
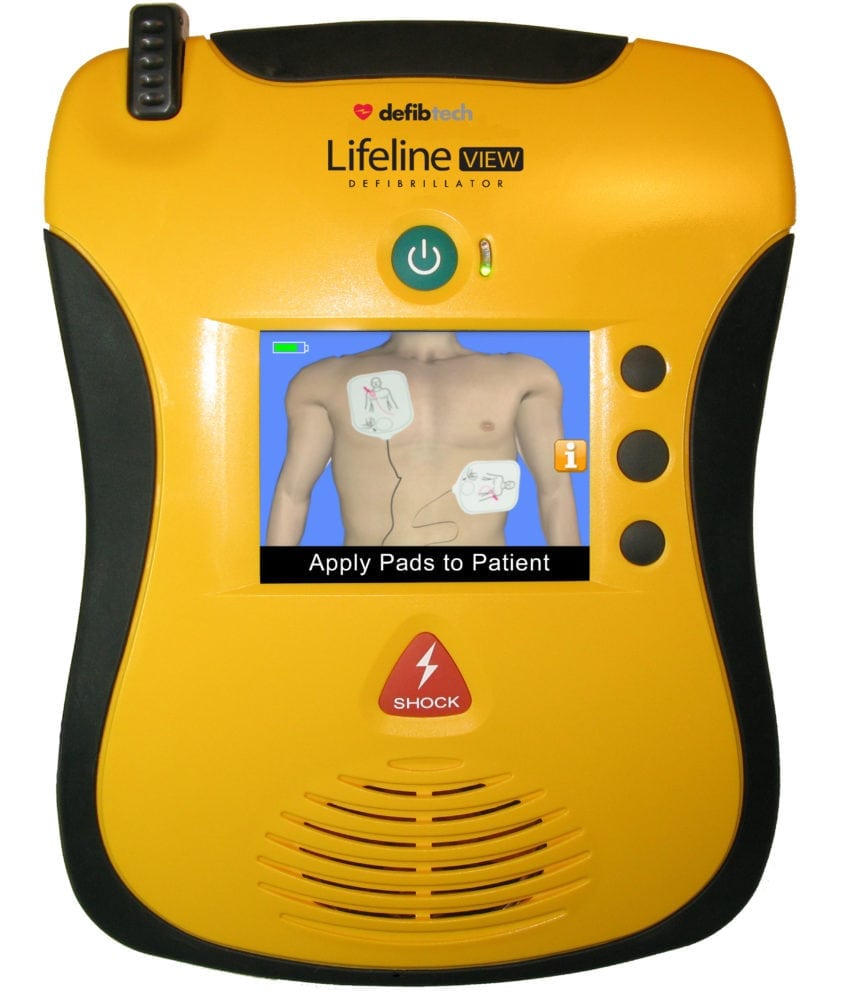
If rescue breathing is chosen, the video will show you how and when to provide rescue breaths. The federal watchdog agency said it’s received 28,000 reports of AED failures in the last five years, yet 300,000 Americans could benefit from treatment from the devices every year. The Defibtech VIEW AED allows the user to choose compression-only CPR or compressions with rescue breathing. The 100b is one of the most popular defibrillators on the market.

Statements that apply to all trade names/model numbers listed. Defibtech 100b Reviver AED for sale (888) 228-7564 Click the link to view product information and pricing. The watchdog agency in November announced its External Defibrillator Improvement Initiative aimed at improving the safety and efficacy of automated external defibrillators. The Lifeline/ReviveR ECG is referred to as the DDU-2450 from this point forward in this manual. In June 2010, the AED manufacturer initiated a voluntary recall of 5,418 DBP-2800 battery packs for the Lifeline AED and ReviveR AED products.ĪEDs have fallen under more FDA scrutiny recently. This is the second recall in the last 12 months affecting the same products. Most sudden cardiac deaths occur outside of the hospital.
#REVIVER AED HOW TO#
The company said its customers should keep their AEDs in service while the company sends out instructions on how to perform a corrective software upgrade. Automated external defibrillators (AEDs) are an important lifesaving technology and may have a role to play in treating workplace cardiac arrest. Defibtech also warned that conditions that cause the defect are not detectable by the AED’s periodic self test. Most Popular RespondER Premium CPR/AED Pack with RespondER Mask in nylon pouch 29.95 ADD TO CART 2 Rescuer RespondER PREMIUM CPR/AED Pack with Masks in Hard Case 39. We carry single CPR kits for sale as well. Though failures are rare, the affected AEDs may cancel a life-saving shock during the device’s charging process, the company said. Our AED/CPR Rescue Kits are designed for demanding situations, and contain the finest materials we could find.
#REVIVER AED SERIES#
The Guilford, Conn.-based company said the recall affects only DDU-100 Series AEDs shipped with 2.004 software or earlier. The FDA classified the recall as Class I because it believes the products’ possible malfunction could cause serious injury or death. The recall notice, which affects 65,885 AEDs distributed in the United States, applies to AEDs marketed under the Lifeline AED and ReviveR AED brands, the company said today. Defibtech LLC initiated a global recall for two of its semi-automatic external defibrillators.


 0 kommentar(er)
0 kommentar(er)
